How to Prepare 10 M Koh Solution
Label the bottle and. Procedure to make 100 ml of KOH 20 wv solution.
Transfer the specimen small pieces to the drop of KOH and cover with glass.
. Dissolve about 6 g of potassium hydroxide in 5 ml of water. Make Your Own Solution for These Projects. 1000 ml of Potassium Hydroxide will contain 1000 x 45686813 6552 grams of KOH.
Add a small volume of distilled deionized water to dissolve the salt. Place the NaCl in a 1-liter volumetric flask. Add 50 ml distilled water and mix until the chemical is completely dissolved add remaining distilled water and make the volume 100 ml.
To prepare a 5M HCl solution you need to know the concentration of your source of HCl. Stir until the crystals completely dissolve. Add remaining distilled water to make the volume 100ml.
Substituting the known values m 0350 040 L m 014 mols. As an example to make 100 ml of 10 NaCl table salt solution use the previous formula to find out how much NaCl you need. What volumeL of 3M KOH solution can be perpared by diluting 05 L of M KOH solution.
10 KOH option would be produced by adding 10g of KOH crystals to 50 ml of sterilized water. If there is no further clarification in the specification of the 10 like 10vol or 10mol then this should indicate a mass fraction. 5611 grams of KOH is equal to 1 mole.
1 gram of KOH will be equal to 15611 moles. If the purity is 85 then you must take 345g and dissolve it in 100 mL water. Mass 014 mols 561056 gmol.
3 How would you make 1 liter of a 2 M KCl solution. After the solid is completely dissolved dilute the solution to a final volume with deionized distilled water. Slowly add the 40 grams of pellets a few at a time adjusting the.
Bioassay Test for Toxicity. Place a drop of KOH solution on a slide. Put about 50 ml of water in a 150-ml or 250-ml Pyrex beaker with a magnetic stir bar and start it stirring on a magnetic stirrer.
10 KOH solution is made by adding 10g of KOH crystals to 50 ml of distilled water. Dissolve 111 g NaCl in 100 ml of water. Atomic weight of K 10 O 16 H 1.
Procedure of KOH Preparation. Add remaining sterilized water to help make the volume 100ml. Measure 17g solids and dissolve with 30ml deionized water then dilute to 500ml with 95 absolute ethanol transfer to volumetric flask stand for 24h and weigh with 075g potassium hydrogen phthalate.
Add 50 ml distilled water and mix until the chemical iscompletely dissolved add remaining distilled water and make thevolume 100 ml. Now you can make your solution. Procedure to make 100 ml of KOH 20 wv solution.
Click hereto get an answer to your question Calculate the amount of KOH required to prepare 100 mL of 01 M solution. Label the bottle and mark it corrosive. If a different molarity is required then multiply that number times the molar mass of NaCl.
Add 10 ml of glycerol to prevent drying. How do you mix KOH solutions. Weigh 20 g potassium hydroxide KOH pellets.
Add sufficient aldehyde-free ethanol 95 to produce 1000 ml. Fill the flask to the 1 L line. PH - log 10 H 3 O M.
Then you can use this proportion C1V1C2V2 where CConcentration in Mmolesliter and VVolumeliters. H 3 O ½10010-14 M 210010-1 M1 1 410010-1 M3210-15 M10010-14 M 2 ½100 112810-13 M 10310-13 M. Weigh 20 g potassium hydroxide KOH pellets.
Stir before the crystals completely dissolve. We will need to dilute 1372 mL of 73 AlCl36H2O to a final volume with deionized distilled water. Show activity on this post.
Transfer the chemical to a screw-cap bottle. Place the slide in a petri dish or another container with a lid together with a damp piece of filter paper or cotton wool to prevent the preparation from drying out. 2 What is the final volume of 010 m Naoh that can be prepared from 250 mL of 030 M Naoh.
1 How much water would I need to add to 500 mL of a 24 M KCl solution to make a 10 M solution. Place specimen in 10 KOH cover with a coverslip and press gently to make a thin mount. 4 What is the molarity of a solution containing 50 moles of KCl in 20 l of solution.
How do I make a 10 KOH solution. Transfer the prepared solution to a. Add 10 ml of glycerol to avoid drying.
Then multiply the number of moles by the molar mass of KOH which is equal to 561056 gmol. How can we Prepare 01 M solution of KOH in. Calculate the number of moles of Potassium Hydroxide present in 6552 g of Potassium Hydroxide solution.
To determine the number of moles given molarity and volume we use the equation m M V volume should be in liters. N10 NaOH Prepare concentrated stock solution Say 50 of NaOH by dissolving equal parts of NaOH pellets 50 gm water 50 gm in a flask Keep it tightly stoppered for 3-4 days Use the clean supernatant liquid for preparing N10 solution Approximately 8 ml of this stock solution 50 is required per litre of distilled water. Grams of NaCl 10 x 100 100 10 111 g.
30g pure KOH in 100 mL water 30 solution. According to ckoh m o-benzene 1000 v koh M o-benzene reverse push v koh 734ml. 1000 ml of Potassium Hydroxide will contain 6552 grams of KOH.
Allow the solution to stand in a tightly-stoppered bottle for 24 hours. Thus 10 of the mass of the solution is supposed to be K O H so you would dissolve 10 g in 90 g ethanol to get the desired solution. Place a drop of 10 KOH in the center of a clean glass slide.
Transfer the chemical to a screw-cap bottle.

Calculate The Molarity Of Koh In Solution Prepared By Dissolving 5 6 G In Enough Water To Fo Youtube

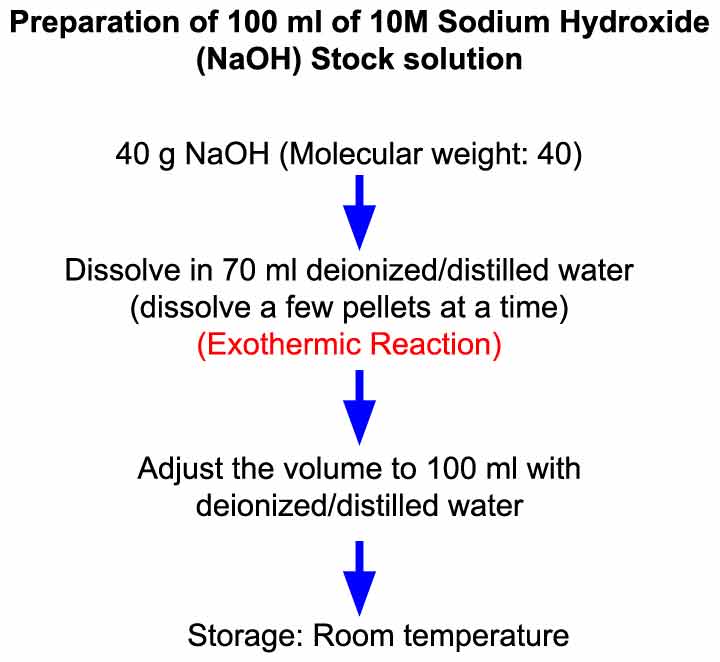
Preparation Of 10 M Sodium Hydroxide Naoh Solution Laboratory Notes


Comments
Post a Comment